Article Text
Statistics from Altmetric.com
Clinical presentation
A woman in her early 20s died suddenly after dinner at a restaurant. She had been served a meal consisting of cream of pea, bruschetta and pasta with wild boar ragu. The young woman suffered first dyspnoea and then vomiting. While she was self-administering epinephrine, friends called the ambulance; emergency personnel made resuscitation attempts and tracheal aspiration from which gastric content was recovered. Despite further administration of epinephrine, the woman remained haemodynamically unstable and died. This death was reported to the competent judicial authority and the restaurant owner was accused of probable negligence in supplying food with a consequent anaphylactic reaction.
Laboratory investigation
Autopsy and postmortem investigations
A complete autopsy was performed 72 hours after death. Vomit and gastric content were taken for analysis. To stop the residual proteolytic activity of pepsin, a portion of the gastric content was neutralised (to pH 7) with sodium hydroxide. All the biological samples were maintained at −20°C until the analysis. During autopsy, specimens from all organs were taken and fixed in formalin and embedded in paraffin. Histological sections were stained with H&E. In addition, immunohistochemical investigation of lung samples was performed using antitryptase antibody. We used 4 µm thick paraffin sections mounted on slides covered with 3-amminopropyl-triethoxysilane (Fluka, Buchs, Switzerland). Pre-treatment was necessary to facilitate antigen retrieval and to increase membrane permeability to antibodies: for fibrinogen, enzymatic digestion with Proteinase K in 20 mM Tris–HCl, pH 8.0 for 15 min (temperature 20°C); for tryptase, enzymatic digestion with proteolytic enzyme (Dako, Copenhagen, Denmark) for 5 min (temperature 20°C). The primary antibody was applied in a ratio of 1:1000 for tryptase and incubated for 120 min at 20°C. The detection system used was the LSAB+ kit (Dako), a refined avidin–biotin technique in which a biotinylated secondary antibody reacts with several peroxidase-conjugated streptavidin molecules. The positive reaction was visualised by 3,3-diaminobenzidine peroxidation, according to standard methods. The sections were counterstained with Mayer’s haematoxylin, dehydrated, coverslipped and observed in a Leica DM4000B optical microscope (Leica, Cambridge, UK).
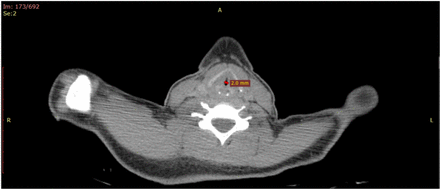
Postmortem CT (PMCT) revealed narrowing of the glottis rim (figure 1). The macroscopic examination showed a swelling of the larynx, and gastric content that had entered the oesophagus and the upper respiratory tract.
Postmortem CT (PMCT): Narrowing of the glottis rim.
Routine histological examination of lung samples revealed congestion, severe acute emphysema, focal cellular eosinophilic peri-bronchial infiltration (figure 2A) and intrabronchial mucus plugs (figure 2B). Other organs were unremarkable except for cerebral oedema. On immunohistochemical investigation of lung specimens, pulmonary mast cells in the bronchial walls and capillary septa were identified and a large number of degranulating mast cells with tryptase-positive material outside the cells were documented. A halo of tryptase positiveness around the mast cells revealed evidence of mast cell degranulation (figure 2C,D).
(A) Focal cellular eosinophilic peri-bronchial infiltration (H&E, ×40); (B) intrabronchial mucus plugs (H&E, ×10); (C,D) degranulating mast cells (starry effect) with tryptase-positive material outside the cells was documented (Ab anti-tryptase). A halo (golden reaction) of tryptase positiveness around the mast cells reveals evidence of mast cell degranulation.
Blood tryptase was determined in postmortem blood with the ImmunoCAP 250 tryptase assay (Thermo Fisher Scientific, formerly Phadia, Uppsala, Sweden). Specific circulating IgEs versus egg and milk allergens were measured in postmortem haemolysed blood by the ImmunoCAP Lab tests (Thermo Fisher Scientific).
The clinical chemistry analyses carried out on the serum sample taken at autopsy gave the following results:
High serum tryptase levels (57.3 µg/L).
High values of antibodies against the most important egg and milk allergens. The following antibodies were detected: white egg (0.45 kUA/L), yolk (0.32 kUA/L), milk (17.2 kUA/L), alpha-lactalbumin (2.05 kUA/L) and beta-lactoglobulin (2.0 kUA/L).
Experimental data
The molecular study was performed on biological samples collected during autopsy (vomit, gastric content with and without neutralisation) and foods (cream of asparagus and cream of pea, commercial bread and restaurant-made bread, commercial pasta, pheasant and wild boar ragu) considered as a possible source of the offending allergens.
According to friends’ statements, cream of asparagus, pheasant ragu and restaurant-made bread were not consumed by the young woman, but they were collected for their similarity to the other food served, and on the hypothesis of a possible food exchange.
To improve the possibility of detecting allergens, which could be present in very low quantities, biological samples and foods were freeze-dried (Edwards RV8; BOC Edwards, Burgess Hill, UK), and weight loss was registered accurately. Before dehydration, a portion of gastric content was explored to separate solid elements, such as possible residues of undigested bread.
The search and quantification of milk proteins were performed with the RIDASCREENFAST Milk ELISA kit (R-Biopharm, Darmstadt, Germany). The limit of quantification is 2.5 mg/kg of total milk proteins. To each sample (0.5 g) was added 2 mL of diluted Extractor 2 solution; further extraction steps and quantification were carried out following the instructions given in the kit. Each sample was extracted in duplicate.
Egg white proteins were searched and quantified by the RIDASCREENFAST Ei/Egg protein kit (R-Biopharm). The limit of quantification is 0.13 mg/kg of egg white proteins.
This search was considered particularly important due to the presence of egg white powder in the kitchen as a possible source of contamination. Ten millilitres of diluted Allergen Extraction buffer was added to samples (0.5 g); further extraction steps and quantification were carried out following the instructions given in the kit. Each sample was extracted in duplicate.
The samples analysed with both ELISA tests were the following: cream of asparagus, cream of pea, restaurant-made bread, commercial bread and biological samples (vomit, gastric content as such, neutralised gastric contents and solid elements from gastric content). Pasta was not included since preliminary tests showed the absence of milk and egg allergens.
Presence of allergenic proteins in biological fluids
The content of allergenic proteins was quantified in undigested bread samples as such and in freeze-dried biological samples in the other cases to increase the possibility of detection and quantification of egg and milk proteins. Values were then calculated on the original samples, using the weight loss registered during freeze-drying; multiplying factors ranged between three and five times (table 1).
Quantification of milk and egg proteins in biological samples collected during autopsy
Presence of allergenic proteins in foods collected at the restaurant
The content of allergenic proteins was quantified in foods similar to those presumably consumed by the victim at the restaurant where anaphylaxis occurred (table 2).
Quantification of milk and egg proteins in foods collected at restaurant
Discussion
The occurrence of fatal food anaphylaxis is quite rare,1 but the severity of the symptoms and the possible irreversible conclusion make these events particularly dramatic.
This paper describes the analytical approach used to identify the cause of a fatal anaphylactic shock which occurred in a young woman, known for her severe allergy to milk and eggs, after dinner in a restaurant.
Different clinical chemistry analyses were carried out in this study. In the serum sample taken at autopsy, high serum tryptase levels (57.3 µg/L) were detected. The usual reference level for positivity is >13.5 µg/L, but this value could not be directly applicable to postmortem conditions, also considering that serum cannot be obtained by haemolysed postmortem blood specimens. However, scientific societies involved in Allergy and Clinical Immunology recommend the measure of serum tryptase, which may be helpful in confirming a diagnosis of anaphylaxis or ruling out other causes.2 3 Considering that in allergic subjects sensitisation is present when IgEs are above 0.1 kUA/L, in this case the analyses were positive for egg white (0.45 kUA/L), yolk (0.32 kUA/L), milk (17.2 kUA/L), alpha-lactalbumin (2.05 kUA/L) and beta-lactoglobulin (2.0 kUA/L).
As illustrated in table 1, all biological samples were free from egg allergens but contained milk proteins. The concentrations of milk allergens decreased from vomit (2.05±0.44 mg/kg) to the untreated gastric sample (0.94±0.08 mg/kg). It is interesting to note that the neutralisation of gastric content blocked proteolysis, which proceeded significantly even in post mortem.
The milk protein concentration in solid materials (undigested bread) was higher than that found in the whole corresponding sample.
The total amount of milk protein in biological samples was calculated by multiplying the concentration by the total weight collected. The total amount of protein obtained from biopsy samples is therefore equivalent to 0.443 mg. This value is certainly underestimated since human gastric content can vary between 0.5 and 1.5 L.
The results show clearly that the young woman consumed food containing milk proteins, while no exposure to egg derivatives has been identified, as shown by the absence of positivity (data below limit of quantification, LOQ) in all biological fluids also when analysed as freeze-dried derivatives.
Regarding the presence of allergenic proteins in foods (table 2), commercial bread and wild boar ragu were free from egg white and milk allergens (below LOQ). A low amount of egg white proteins was found in the cream of pea (1.76±0.39 mg/kg) and pheasant ragu (0.10±0.01 mg/kg). In both vegetable creams, a low quantity of milk allergens was measured (approximately 0.65 mg/kg) while, as indicated also by the personnel, the restaurant-made bread contained a very high quantity of milk proteins (approximately 1.2 mg/g).
By analysing the foods involved in this event, it was possible to focus on two main problems:
Although they did not contain eggs or milk as ingredients, the vegetable creams were contaminated in both cases by milk proteins; the cream of pea also contained traces of egg protein. However, it must be emphasised that the measured amounts were minute and quantifiable only after freeze-drying and concentration of the samples.
The restaurant-made bread contained, as an ingredient, milk proteins and this was confirmed by the very high concentration measured.
Based on these results, two hypotheses could be formulated, namely, that the victim had consumed (1) a large amount of accidentally contaminated cream of pea, and (2) there had been a mistake in the preparation of bruschetta, where bread produced in the restaurant had been used instead of commercial bread.
To solve this doubt, calculations were made on the total content of milk proteins measured in the biological fluids, corresponding to 0.443 mg. This value was certainly lower than the original one because of proteolysis and for the only partial collection of gastric content. If the cream of pea were responsible for the anaphylactic shock, the young woman would have had to consume about 670 g of this product. Even in the case of asparagus cream, the necessary quantities would be equivalent. This intake is not justifiable due to the small portion usual for an appetiser. On the contrary, the same total of milk proteins found in biological fluids could have been supplied by 0.360 g of restaurant-made bread. In conclusion, even though the cream of pea may have contributed to the allergic reaction, responsibility for the event must be associated with the substitution of (milk-free) commercial bread with the one produced on site that contained milk proteins.
Conclusion
Although several histological and immunohistochemical investigation techniques are at disposal,4–6 a specific protocol for postmortem molecular analyses is still lacking. Fatal food anaphylaxis is quite rare, but a suitable approach is necessary to identify the allergen and the food involved, in order to recognise the person/s responsible for possible negligent serving or food handling.
This paper shows clearly that the postmortem examination in the case of anaphylactic shock needs specific protocol and particular attention must be paid to the inhibition of proteolysis, which lasts for a long time even post mortem. This approach is essential to avoid the digestion of allergenic proteins that can no longer be measured by commonly used tests.
Take home messages
The postmortem identification and quantification of allergens involved in suspected anaphylaxis is difficult.
A specific protocol for the postmortem investigation of fatal food-associated anaphylaxis, based on both autopsy and laboratory tests, should be followed to identify the offending allergen.
It is important to inhibit gastrointestinal enzymes in order to avoid the proteolysis of allergenic proteins that can no longer be measured by the usual immunochemical tests.
Questions for discussion
How does one identify anaphylaxis as a cause of death?
How does one identify offending allergens post mortem, if allergy is suspected?
When is it necessary to identify and quantify the potential protein allergens involved in a case of fatal anaphylaxis?
Footnotes
Handling editor Tahir S Pillay.
Contributors All authors contributed to the study conception and design. Material preparation, data collection and analysis of foods and biological samples were performed by FC, CDL and SB. MDP and ET conducted the postmortem examination and clinical assays. PR coordinated the experimental work and wrote the draft of the manuscript. All authors read and approved the final manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; internally peer reviewed.
Linked Articles
- Grand rounds commentary
- Grand rounds commentary