Article Text
Abstract
Aims An increasing number of small pulmonary nodules are being screened by CT, and an intraoperative diagnosis is necessary for preventing excessive treatment. However, there is limited literature on the frozen diagnosis of small sclerosing pneumocytomas (SPs). In particular, tumours smaller than 1 cm are challenging for pathologists performing intraoperative frozen diagnosis.
Methods In total, 230 cases of SP were surgically resected between January 2015 and March 2019 at Shanghai Chest Hospital, and of them, 76 cases were smaller than 1 cm. The histology and clinical information of these 76 cases (33.0%, 76/230) were reviewed retrospectively, 54 cases of which were diagnosed intraoperatively, and the pitfalls were summarised. All diagnoses were confirmed on permanent sections and immunohistochemical sections.
Results Histologically, 78.9% (60/76) of the small SP was dominated by one growth pattern, and solid and papillary growth pattern were the most commonly misdiagnosed circumstances. The rate of intraoperative misdiagnosis of these SP smaller than 1 cm was 11.1% (6/54).
Conclusions The main reason for misdiagnosis was failure to recognise the dual cell populations and the cellular atypia. Diagnostic clues include the gross morphology, the presence of dual-cell populations and a hypercellular papillary core, foam cell accumulation in glandular spaces and haemorrhage and haemosiderin on the periphery. In spite of awareness of pitfalls some cases may still be essentially impossible to diagnose on frozen section.
- lung
- pathology
- surgical
- lung neoplasms
- adenoma
- pleomorphic
Data availability statement
All data relevant to the study are included in the article.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Sclerosing haemangioma was classified as an adenoma in the 2015 WHO classification and was renamed sclerosing pneumocytoma (SP).1 Cubical surface cells and stromal round cells are the key features of SP.2 On CT images, they appear as solid, well-defined round or oval lumps with low CT values. Although there are rare cases of regional lymph node metastasis, the prognosis is good after complete resection.3
The diagnosis of SP is difficult on frozen sections, sometimes even on paraffin sections, and the diagnosis of small SP is more difficult. There is limited literature on the frozen diagnosis of small SPs. In a 2004 paper, the frozen diagnosis of 183 consecutive pulmonary nodules smaller than 1.5 cm in diameter, including SP, suggests that the diagnostic accuracy of frozen sections was significantly better in nodules larger than 1.0 cm in diameter.4 Recently, the characteristics of frozen diagnoses of 59 cases of routine SP (0.6–6.5 mm) have been reported.5 However, we do not know much about the morphology of small SP ≤1 cm (small SP). Do they still more or less maintain the four characteristic patterns?
With the wide use of high-resolution CT, an increasing number of small pulmonary nodules have been identified. Intraoperative frozen section diagnosis is important for separating benign nodules from malignant tumours. The motivation behind this study was the intraoperative misdiagnosis rate of small SPs in our department, which was much higher than that of the larger SPs from January 2015 to March 2019. These small SPs are misdiagnosed as malignant tumours such as small adenocarcinoma, carcinoid tumours, salivary gland tumours and metastatic cancer in intraoperative frozen sections and may therefore result in overtreatment.6 The lack of characteristic growth patterns and the challenge of identifying dual-cell populations on frozen sections make the diagnosis of small SP difficult.
Methods
In total, 230 SP patients underwent surgical resection in Shanghai Chest Hospital from January 2015 to March 2019. All cases of small SP, which accounted for 33.0% (76/230), were reviewed retrospectively (figure 1A), among which 54 cases had intraoperative diagnosis. And the final diagnosis was confirmed by paraffin section and immunohistochemistry (IHC). All immunohistochemical sections were performed by SP method, and markers included Pan cytokeratin, vimentin, TTF-1 and Ki-67. CD56, chromograninA and synaptophysin are also included in the diagnosis of some difficult cases. The dilutions and antigen retrieval methods were shown in table 1.
Distribution and growth pattern of 76 small sclerosing pneumocytomas (SPs) in the surgical resection SPs. (A) Of the 230 patients with SP who underwent surgery, 33.0% had small SPs. (B) Forty-two per cent of the 76 small SP cases were dominated by a solid growth pattern.
Antibodies used in the immunohistochemistry
All small SPs were re-diagnosed by two pathologists on the basis of original frozen section slides, permanent section H&E slides and immunohistochemical sections. According to the growth patterns, all cases were divided into two groups: (1) multiple-growth-pattern group: two or more growth patterns, with each component accounting for less than or equal to 50%; and (2) dominant-growth-pattern group: nodules dominated by one pattern greater than 50%. The latter were divided into four subgroups according to the dominant growth pattern. The pathological and imaging features and the diagnostic pitfalls in frozen sections of the small SP were summarised and analysed.
Results
Clinical and imaging features
The size distribution of surgically resected SP cases is listed in figure 1A. Small SPs accounted for 33.0% (76/230) of all SPs; 36.5% (84/230) was between 1.1 cm and 2.0 cm in diameter, 16.1% (37/230) was between 2.1 cm and 3.0 cm, and 14.3% (33/230) was larger than 3.0 cm. All cases were confirmed by paraffin and IHC diagnosis, the epithelial cells were strongly positive for Pan cytokeratin, the round stromal cells were positive for vimentin, and both of them were positive for TTF-1.
Small SPs were mainly found in female patients (90.7%). The age ranged from 34 to 79 years, and 80.3% (61/76) of patients with small SPs were 40–69 years old. A total of 30.3% of small SPs were located in the left lower lobe; 34.2% (26/76) were single nodules, while 61.8% (47/76) were concomitant with other thorax lesions, such as pulmonary adenocarcinoma, thymoma or granuloma.
Three cases (3.9%) presented as a larger SP with satellite small SP nodules in the same lobe; in one of these cases, round stromal cells and sclerosis components had metastasised to the regional lymph nodes. During follow-up, this patient has shown no recurrence or distant metastasis to date (>30 months). The demographic characteristics and clinical information of the 76 patients with small SPs are presented in table 2.
Demographic characteristics and clinical information of the 76 patient with small sclerosing pneumocytoma
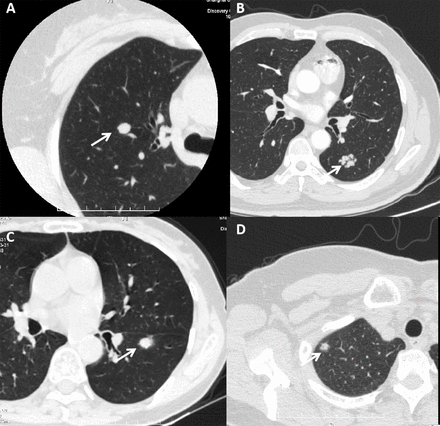
Seventy-five cases of small SP with preoperative CT at Shanghai Thoracic Hospital presented peripheral lesions. The main signs on CT were well-circumscribed solid nodules (81.3%, 61/75) (figure 2A). Microcalcifications and enhancement effects were not uncommon. One small SP was lobulated, but there was no cartilage or fat density of hamartoma (figure 2B). Notably, 18.7% of small SPs (14/75) displayed unusual imaging characteristics and four of them presented as pure or mixed ground-glass nodules. Pleural traction was identified in two cases, and these tumours were suspected to be malignant nodules by CT (figure 2C,D).
CT findings of small sclerosing pneumocytoma nodules. The white arrows point to the lesions. (A) Well-circumscribed solid nodules with an enhancement effect. (B) Lobulated structure similar to a hamartoma. (C) Small nodules with pleural traction suspicious for a malignant tumour. (D) Mixed ground-glass nodules, which need to be differentiated from primary lung cancer nodules.
Intraoperative frozen section diagnosis
Of the 76 small SPs, 16 (21.1%) were classified into the multiple-growth-pattern group, and 60 (78.9%) were classified into the dominant-growth-pattern group: 32 cases were solid, 13 cases were papillary, 10 cases were sclerosing and 5 cases were haemorrhagic (figure 1B).
The intraoperative misdiagnosis rate of small SPs (≤1 cm) in our department was 11.1% (6/54), which was much higher than that of the larger SPs (>1 cm) (1/154, 0.006%). The 54 cases according to the intraoperative diagnosis were divided into four groups (table 3). The solid growth pattern was the most common pattern in both the multiple-growth-pattern group and the dominant-growth-pattern group, and it was also the main reason for misdiagnosis.
Microscopic features of 54 cases of small sclerosing pneumocytoma with an intraoperative frozen diagnosis
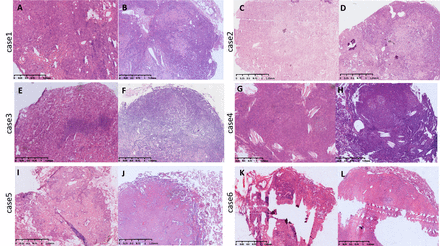
Specifically, in the multiple-growth-pattern group, five small SPs were diagnosed as SP during the operation, and the other five small SPs were suspected to be SP or other benign or low-grade malignant tumour without an accurate diagnosis. In the dominant-growth-pattern group, two cases dominated by the solid pattern and one case dominated by the papillary pattern were over-diagnosed as invasive adenocarcinoma (figure 3A–F). One case dominated by a solid pattern and two cases dominated by a sclerotic pattern were misdiagnosed as inflammatory lesions (figure 3G–L). Six solid-dominated cases, two papillary-dominant cases and one haemorrhagic-dominant case were given a deferral diagnosis.
Cases misdiagnosed on frozen section (H&E stain 20×). (A–F) Two cases dominated by the solid pattern and one case dominated by the papillary pattern were over-diagnosed as invasive adenocarcinoma. Gland and papillary structures, and the cellular atypia were important factors leading to misdiagnosis. (G–H) One case dominated by a solid pattern with lots of inflammatory cells and necrosis was misdiagnosed as inflammatory lesions. (I–L) Two cases dominated by a sclerotic pattern with a very small number of stromal round cells were misdiagnosed as inflammatory lesions.
Morphological characteristics and diagnostic pitfalls of frozen sections
Microscopically, the two cellular components are difficult to identify
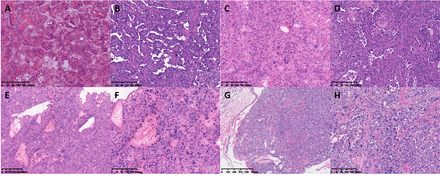
The solid-dominant subtype consisted of sheets of small round cells with few glandular or papillary structures (figure 4A–D). Misdiagnosis includes carcinoid tumors.7 Even one case was still difficult to distinguish between carcinoid and SP in the paraffin section (figure 4E–H). Occasionally, carcinoid components were present in SP lesion (figure 4I–L). Carefully looking for the cubical epithelial cell-lined glands and underlying oval stromal cells are key points. Usually the oval stromal cells are round with transparent cytoplasm. Foam cells in the glandular cavity are an important clue for the diagnosis of SP.
Solid growth pattern. (A) Round cells arranged in sheets and a small number of glandular spaces in the frozen section (H&E stain 40×). (B) Bronchiolar structure and foam cells in some areas in the permanent section slide (H&E stain 40×). (C) Glandular spaces were positive for keratins (immunohistochemical stain 40×). (D) The epithelial cells and stromal round cells were both positive for TTF-1 (immunohistochemical stain 40×). (E) Frozen section slide of a nodule of a typical carcinoid tumour (H&E stain 20×). (F) Papillary growth pattern and glandular spaces were much like sp (H&E stain 40×). (G) Permanent section slide of the typical carcinoid case (H&E stain 40×). (H) So-called stromal round cells were negative for vimentin (immunohistochemical stain 40×). (I) A small foci of cells were arranged in cords or trabeculae in frozen section slides of SP(H&E stain 20×). (J) Local high magnification (H&E stain 40×). (K) The morphological characteristics of neuroendocrine tumours could be observed in permanent section slides (H&E stain 40×). (L) The typical carcinoid component was positive for CD56 (immunohistochemical stain 40×).
It is difficult to distinguish the papillary structure of SP from that of adenocarcinoma
Regarding the papillary-dominant subtype, one case was misdiagnosed as poorly differentiated carcinoma because of epithelial cell atypia and a sclerosing papillary core, similar to that of papillary invasive carcinoma. Cellular atypia is an important misdiagnosis pitfall, although quite uncommon (figure 5). Deferral diagnoses were made in two cases because of the papillary structure could not be differentiated from adenocarcinoma. The key point is that unlike the fibrovascular core of papillary adenoma or adenocarcinoma, the papillary core of SP is hypercellular or significantly collagenous. In this circumstance, gross examination would be of great help; SP is well circumscribed and tends to shell out during operation, while invasive adenocarcinoma is not.
Papillary growth pattern and atypia of tumour cells and tissue structure. (A) A papillary structure and atypical epithelial cells can be observed on the frozen section. This appearance is similar to that of papillary carcinoma (H&E stain 100×). (B) The papillary core is not fibrovascular but is rich in collagen or cellular components in the permanent section slide (H&E stain 100×). (C) The small sclerosing pneumocytoma epithelial cells of the glandular spaces were atypical in the frozen section (H&E stain 100×). (D) the tumour still needs to be distinguished from adenocarcinoma in the permanent section slide (H&E stain 100×). (E) Frozen section slide of an nodule whose epithelial cells and stromal round cells were both atypical (H&E stain 40×). (F) Local high magnification (H&E stain 100×). (G) The two kinds of cells and haemorrhagic area were similar to those of metastatic chorionic carcinoma (H&E stain 40×). (H) Pleomorphic nuclei in high magnification (H&E stain 100×).
Sclerosing stroma made correct diagnosis more difficult
There were five sclerosis-dominant cases, and two were misdiagnosed as inflammatory lesions because collagen nodules associated with bronchial epithelial hyperplasia or metaplasia, sometimes accompanied by mucus secretion, and peripheral lymphocyte infiltration caused misdiagnosis. Only a few round stromal cells were identified on frozen section (figure 3I,J). A careful search for round cells in the collagenous stroma is the key point.
Fail to recognise the haemorrhage or misdiagnose as haemangioma
Four cases showed a dominant haemorrhagic pattern which fewer epithelial cells identified. One case was delayed because on frozen sections, only cavities without blood cells were recognised, which made the whole tumour structure appear loose, although blood pools of different sizes under the microscope strongly indicated SP in the permanent section slides. Unlike other subtypes, spindle stromal cells are more common in haemorrhage dominant pattern. Therefore, this type of tumour should be differentiated from fibroblast proliferation or meningioma-like hyperplasia nodules.
Discussion
SP has a good prognosis with simple surgical resection,8 and an accurate intraoperative diagnosis could help to avoid unnecessary lobectomy, lymph node dissection or even a second operation.4 Because knowledge of the pathological features of small SP is limited, we reviewed 76 cases of small SP and summarised the pathological features and diagnostic pitfalls on frozen sections.
The four characteristic types of growth patterns and the dual-cell types are still the key pathological features of small SP, although they may appear in different proportions. When the tumour is dominated by one pattern, other clues such as glandular space, two cell components with transparent cytoplasm and eosinophilic cytoplasm mixed together instead of monoform, cellular or collagen papillary axis, foam cell foci1 and haemosiderin in macrophages on the periphery of the tumour are helpful for the diagnosis.
Other factors that can aid in intraoperative diagnosis are gross morphology or imaging. Grossly, the section of the larger mass of SP is greyish yellow or dark red with a hard texture, which is different from the grey or greyish red colour of adenocarcinoma. It should be noted that few small SP cases with dark-red haemorrhagic and hard areas need to be differentiated from metastatic choriocarcinoma. In practice, when it is difficult to diagnose by histology on frozen sections, the gross morphology is helpful. Other pitfalls include single small SP maybe mistaken for metastatic cancer nodule because of well-circumscribed, and for the high frequency of coexisting tumours, especially concomitant invasive adenocarcinoma. For imaging, small SP has a smoother boundary, but lung adenocarcinoma appears as an infiltrative growth pattern.9 Occasionally, CT images may be misleading; when the small SP is accompanied by inflammation or granulomatous lesions, it appears as a solid nodule with pleural traction that resembles a malignant tumour on CT.
In our cohort a large number of the small nodule cases (47/76) were not single nodules but associated with other tumour types including several with adenocarcinomas. For SP cases with adenocarcinoma, the surgical approach mainly depends on the adenocarcinoma, and the misdiagnosis would not affect the treatment strategy so much. For single SP nodule, the misdiagnosis would affect the types of surgery. Among the three cases we misdiagnosed as invasive adenocarcinoma, one case is a single nodule that was given a regional lymphadenectomy following a right upper lobectomy. The other two combining with invasive adenocarcinoma cases did not result in a change in surgical procedure. However, if the misdiagnosis cannot be corrected on paraffin sections, the presence of adenocarcinoma in different lobes will lead to tumour, node, metastases staging changes, which in turn affects postoperative treatment.
Although we provided with some clues for intraoperative diagnosis of SP, especially focusing on small sized SP, there still remain cases which may be impossible to diagnose on frozen section and may still be difficult on permanent section.
Conclusion
Small SPs maintain four growth patterns that vary in predominance, but most are dominated by one growth pattern. The rate of intraoperative frozen section misdiagnosis of small SPs was higher than that of ordinary sized SPs. A solid growth pattern and occasionally nuclear atypia were the main factors leading to misdiagnosis. In addition to the four growth patterns, foam cells foci, mixed cell components and hypercellular papillary core are also diagnostic clues for SP. Furthermore, morphology, gross examination and imaging should be considered comprehensively for proper intraoperative diagnosis of small SP. However, there will remain cases which may be impossible to diagnose on frozen section and may still be difficult on permanent section.
Take home messages
The rate of misdiagnosis of small sclerosing pneumocytomas (SPs) during the operation was higher than that of ordinary sized SPs.
The solid growth pattern and papillary pattern were the main factors leading to misdiagnosis.
A variety of morphological features, such as foam cell foci, mixture cellular components and hypercellular papillary core, can provide diagnostic clues.
Difficult diagnoses still rely on paraffin and immunohistochemistry.
Data availability statement
All data relevant to the study are included in the article.
Ethics statements
Patient consent for publication
Ethics approval
The study was approved by the Ethics Committee of Shanghai Chest Hospital and conducted in accordance with the Declaration of Helsinki of 1964 and its later amendments.
Footnotes
Handling editor Dhirendra Govender.
Contributors ZS conceived and designed the study, performed the data analyses and wrote the manuscript; JS collected data and helped perform the analysis with constructive discussions; LZ collected data; HT collected data; JZ contributed to the conception of the study; YH, who is the corresponding author, helped to modify the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.