Article Text
Abstract
Aims Intrahepatic cholangiocarcinoma (iCCA) is a diagnosis of exclusion that can pose a challenge to the pathologist despite thorough clinical workup. Although several immunohistochemical markers have been proposed for iCCA, none of them reached clinical practice. We here assessed the combined usage of two promising diagnostic approaches, albumin in situ hybridisation (Alb-ISH) and C reactive protein (CRP) immunohistochemistry, for distinguishing iCCA from other adenocarcinoma primaries.
Methods We conducted Alb-ISH and CRP immunohistochemistry in a large European iCCA cohort (n=153) and compared the results with a spectrum of other glandular adenocarcinomas of different origin (n=885). In addition, we correlated expression patterns with clinicopathological information and mutation data.
Results Alb-ISH was highly specific for iCCA (specificity 98.8%) with almost complete negativity in perihilar CCA and only rare positives among other adenocarcinomas (sensitivity 69.5%). CRP identified the vast majority of iCCA cases (sensitivity 84.1%) at a lower specificity of 86.4%. Strikingly, the combination of CRP and Alb-ISH boosted the diagnostic sensitivity to 88.0% while retaining a considerable specificity of 86.1%. Alb-ISH significantly correlated with CRP expression, specific tumour morphologies and small or large duct iCCA subtypes. Neither Alb-ISH nor CRP was associated with iCCA patient survival. 16 of 17 recurrent mutations in either IDH1, IDH2 and FGFR2 affected Alb-ISH positive cases, while the only KRAS mutation corresponded to an Alb-ISH negative case.
Conclusions In conclusion, we propose a sequential diagnostic approach for iCCA, integrating CRP immunohistochemistry and Alb-ISH. This may improve the accuracy of CCA classification and pave the way towards a molecular-guided CCA classification.
- CHOLANGIOCARCINOMA
- Biliary Tract
- Diagnostic Techniques and Procedures
- Pathology, Molecular
- IMMUNOHISTOCHEMISTRY
Data availability statement
Data are available upon reasonable request. The datasets generated during and/or analysed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- CHOLANGIOCARCINOMA
- Biliary Tract
- Diagnostic Techniques and Procedures
- Pathology, Molecular
- IMMUNOHISTOCHEMISTRY
WHAT IS ALREADY KNOWN ON THIS TOPIC
Intrahepatic cholangiocarcinoma (iCCA) is challenging to diagnose due to the lack of specific morphological and immunohistochemical features.
Previous studies have suggested the diagnostic potential of C reactive protein (CRP) immunohistochemistry and albumin in situ hybridisation (Alb-ISH), but the combination of these methods has not been tested so far using systematic investigations across CCA subtypes and various metastatic adenocarcinomas from different anatomical sites.
WHAT THIS STUDY ADDS
This study demonstrated that Alb-ISH is highly specific for iCCA with almost complete negativity in perihilar CCA (pCCA).
In addition, CRP was found to be highly sensitive for iCCA.
The combined use of CRP and Alb-ISH proved to be reliable for diagnosing iCCA.
Alb-ISH was associated with histological subtyping (small duct/large duct) and molecular profiles.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The study proposes a novel sequential diagnostic approach, incorporating CRP immunohistochemistry and Alb-ISH, to pathologically confirm iCCA at a high accuracy level.
Alb-ISH may serve as a potential biomarker for identifying targetable molecular alterations and may also be used for distinguishing iCCA from pCCA.
Introduction
Cholangiocarcinoma (CCA) constitutes a heterogenous group of malignant tumours with features of cholangiocyte differentiation and grim prognosis.1 According to anatomical location, CCA is categorised as intrahepatic CCA (iCCA), perihilar CCA (pCCA) or distal CCA (dCCA).2 This definition is still the foundation for preoperative management and surgical procedure.3 4 However, with regard to tumour biology, the distinction between iCCA and intrahepatic pCCA is somewhat artificial and of less relevance for systemic treatment decisions. Based on histological appearance and in good correlation with molecular characteristics, iCCA is subtyped into a small or large duct phenotype, the latter of which both morphologically and molecularly more closely resembles pCCA (and dCCA) than its small duct type counterpart.5–7
Although iCCA represents the second most common primary liver cancer, its definite diagnosis may represent a challenge for clinicians and pathologists and in some cases, it remains a diagnosis of exclusion. Histopathological or immunohistological evaluation can be suggestive for metastatic adenocarcinoma in primaries with distinct phenotypes such as colorectal or lung adenocarcinoma, yet in some cases histomorphology alone is inconclusive. As a number of adenocarcinomas with similar histomorphology, for example, pancreatic or gastric cancer, share the immunohistochemical profile of iCCA, the diagnosis of iCCA can also not reliably be established only by conventional pathological diagnostic tools. Thus, the patient regularly has to undergo extensive clinical and imaging workup to exclude metastatic adenocarcinoma before rendering a definitive diagnosis and subsequent treatment decision.
Several previous studies aimed to identify diagnostic markers for iCCA. Among these, N-cadherin was suggested as a promising candidate, however, its comparatively low sensitivity ranging from 54% to 67% translates into a substantial number of false-negative results making this marker less suitable for routine diagnostics.8–10 In 2017, C reactive protein (CRP) was identified as a potential marker for iCCA diagnosis and was shown to provide sensitivity levels of approximately 75%, while retaining a high specificity of 91% similar to that of N-cadherin.11 Beyond immunohistochemistry, recently albumin in situ hybridisation (Alb-ISH) was introduced as another diagnostic tool for iCCA diagnosis bypassing the immunohistochemical obstacle of signal unspecificity due to the abundance of albumin protein in serum and liver tissue.12 13 Alb-ISH was initially postulated to be nearly 100% sensitive for the diagnosis of iCCA (excluding other primary hepatic lesions).12 However, later analyses relativised this finding with false-negative rates reaching up to 36%.14–16 While the specificity for the distinction against metastatic, non-primary hepatic adenocarcinomas was reportedly near-ideal in most studies, in a recent study by Nasir et al. Alb-ISH was also positive in a substantial subset of tumours from diverse sites, including invasive breast carcinoma or lung adenocarcinoma.17
Building on previous studies reporting isolated CRP immunohistochemistry and Alb-ISH and given the diagnostic shortcomings, in this study we assessed the combined use of both markers for the diagnosis of iCCA based on a clinically well-annotated, large European iCCA patient cohort and more than 800 other adenocarcinomas of different primary sites. As a second objective, we explored, whether Alb-ISH or CRP may echo the anatomic dichotomisation into iCCA and pCCA and aimed to unravel a potential association with large and small duct subtyping of iCCA. Lastly, we investigated if Alb-ISH or CRP guided stratification of iCCAs may yield biologically relevant features, such as patient survival and presence of therapy-relevant mutations.
Materials and methods
iCCA cohort characteristics
For this study, a thoroughly characterised clinical iCCA cohort (n=153) was curated, comprising patients undergoing surgery at the Heidelberg University Hospital between 1995 and 2016. Formalin-fixed and paraffin-embedded histological specimens were retrieved from the pathological archive in collaboration with the tissue bank of the National Center for Tumor Diseases. A diagnosis of iCCA was made based on anatomical tumour location, compatible histomorphology and immunohistochemistry, negative clinical and radiological workup for non-hepatic primaries and in some cases molecular data. Only patients with primary adenocarcinomas (including all variants) or adenosquamous carcinomas without any competing malignancy at the time of diagnosis were included in this study. Hepatocellular carcinomas (HCC), mixed HCC–CCAs and patients who received neoadjuvant therapy were excluded. Tumours were restaged according to the eighth edition of the TNM Classification of Malignant Tumors and classified based on the fifth edition of the WHO Classification of Digestive System Tumours.18 19
Adenocarcinoma cohort characteristics
To assess the specificity of the proposed markers, formalin-fixed and paraffin-embedded tissue specimens of additional adenocarcinomas of various locations were obtained. These comprised 153 cases of pCCA, 126 cases of dCCA, 131 cases of gallbladder adenocarcinoma, 98 cases of colorectal liver metastasis, 212 cases of primary invasive breast carcinoma of no special type, 92 cases of primary pancreatic adenocarcinoma and 73 cases of primary pulmonary adenocarcinoma. Due to the availability of previously established tissue microarrays (TMAs), with the exception of colorectal adenocarcinoma, for assessment of diagnostic specificity, primary adenocarcinomas were used. Analogous to the iCCA cohort, all patients received surgical treatment at Heidelberg University Hospital. An overview of all included adenocarcinoma entities is provided in online supplemental table 1.
Supplemental material
iCCA subclassification
iCCA cases were dichotomised into small and large duct subtypes as per the criteria described by the WHO.18 In brief, criteria that favoured a diagnosis of small duct type iCCA were a peripheral hepatic location, a ductal morphology with slit-like lumen, cuboidal tumour cells and both absence of biliary precursor lesions, mucin extravasation and perineural/lymphatic invasion. In contrast, a diagnosis of large duct-type iCCA was rendered in cases with concomitant biliary intraepithelial neoplasia or intraductal papillary neoplasia of the bile duct, columnar cell morphology, tumourous mucin secretion and perineural/lymphatic invasion. For poorly differentiated tumours, subtype classification was performed in areas of better differentiation. Based on the described criteria, all available whole slide specimens per patient were jointly reviewed by two pathologists with particular expertise in biliary tract cancer (TA and BG) and allocated to either subtype.
TMA construction
For generation of TMAs, 3 µm sections were cut and stained with H&E. Thereafter, representative tumour areas were marked by experienced pathologists followed by extraction of duplicate tissue cores from the donor blocks (diameters: breast TMA: 0.5 mm, CCA/gallbladder/pancreas/lung TMA: 1 mm, colorectal metastasis TMA: 1.5 mm) and embedding into a new paraffin array block using a TMA (Beecher Instruments, Woodland, California, USA). After sectioning, due to rolling, floating or detachment of tissue cores, dependent on the respective TMA and the analysis, a small number of cases were not evaluable (see online supplemental table 1).
In situ hybridisation
In situ hybridisation was performed manually using an RNAscope V.2.5 HD brown kit (Advanced Cell Diagnostics, ACD Bio, Newark, California, USA) according to the manufacturer’s protocol. In brief, tissue samples were cut into 5 µm sections and heated at 60°C for 1 hour. Sections were then deparaffinised through a sequence of xylene and ethanol. Hydrogen peroxide was added to each section for 10 min at room temperature. For target retrieval, the slides were incubated with boiling target retrieval solution for 15 min. Protease plus was applied to each section followed by incubation at 40°C in a humidified oven for 45 min. Hybridisation with specific probes for albumin (Hs-ALB, Cat No. 600941) was performed at 40°C for 2 hours. After six amplification steps, slides were incubated for signal detection with diaminobenzidine (DAB) at room temperature for 10 min. Slides were counterstained in 50% haematoxylin for 1 min and subsequently washed with 0.02% ammonia water for 10 s. Hybridisation signals were analysed using a standard bright-field microscope. Normal liver tissue was used as positive control. Non-neoplastic biliary tract and skeletal muscle tissue provided negative controls.
Hybridisation results were interpreted using a semiquantitative scoring system as described previously, that is, cases were counted positive if 5% or more of the tumour cells exhibited distinct cytoplasmic dot-like staining signals at low magnification.15 Nuclear staining was considered a negative result. Hybridisation results were jointly reviewed by two pathologists (TA and BG) in a blinded manner.
Immunohistochemistry
Immunohistochemistry was performed on an automated immunostainer (Ventana BenchMark Ultra, Ventana Medical Systems, Tucson, Arizona, USA) using the biotin-free OptiView DAB IHC Detection Kit (Ventana Medical Systems). In brief, from the formalin fixed and paraffin-embedded TMA blocks, 3 µm sections were cut, deparaffinised, rehydrated and pretreated with an antigen retrieval buffer (Tris/Borate/EDTA, pH 8.4). After blocking of endogenous peroxidase, the slides were incubated with monoclonal antibodies directed against CRP (clone Y284, Abcam, Cambridge, UK) at a dilution of 1:2000, followed by incubation with OptiView Universal Linker and OptiView HRP Multimer. Visualisation was achieved using DAB Chromogen. Before mounting, slides were counterstained with haematoxylin. Normal liver tissue was used as positive control. Non-neoplastic biliary tract and skeletal muscle tissue provided negative controls.
Immunoreactivity for CRP was evaluated semiquantitatively in a blinded manner using an immunoreactive score (IRS) ranging from 0 (no expression) to 12 (high expression), as previously described.20 In brief, staining intensity was scored on a four-tier grade (0: no staining, 1: weak staining, 2: moderate staining, 3: strong staining) and multiplied by the quantity of stained tumour cells (0: no cells stained, 1: <10% positive, 2: 10%–50% positive, 3: 51%–80% positive, 4: >80% positive) to yield the IRS. Cases with an IRS of ≥1 were counted positive.
Statistical analysis
Differences between two groups were assessed using the Mann-Whitney U test. When quantitative data of more than two groups were compared, the Kruskal-Wallis test (non-parametric analysis of variance (ANOVA)) was performed with false discovery rate (FDR) correction (Benjamini and Hochberg). Categorical variables were analysed using Fisher’s exact test. Survival times were graphed using the Kaplan-Meier method and differences were assessed by the Mantel-Cox log-rank test. The association of two variables was assessed using Spearman’s correlation analysis. Statistical analyses were performed with GraphPad Prism V.8.4 (GraphPad Software, La Jolla, California, USA). P values below 0.05 were considered statistically significant.
Results
Alb-ISH is highly specific for iCCA and almost uniformly negative in pCCA
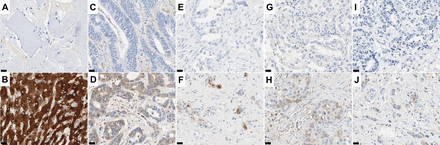
To explore the specificity of Alb-ISH and CRP for a diagnosis of iCCA, we included 885 cases of the most frequent hepatic adenocarcinomas with a glandular phenotype as main differential diagnoses. Among these adenocarcinomas of various sites other than iCCA (figure 1), Alb-ISH yielded almost exclusively negative results with 0% positivity in colorectal liver metastasis (0/88), 1.1% positivity in pancreatic adenocarcinoma (1/87), 1.4% positivity in lung cancer (1/72) and 1.0% positivity in breast cancer (2/206). Analogous results were also observed in gallbladder cancer (1.8% positivity, 2/112) and dCCA (1.6% positivity, 2/126). Albumin expression in the few positive samples was mostly confined to small tumour regions (median percentage of positive tumour cells=20%, among positive samples).
Albumin in situ hybridisation in adenocarcinomas of various sites. Representative microphotographs for albumin in situ hybridisation in adenocarcinomas of various sites, including colorectal liver metastasis ((A), negative), along with positive liver control tissue (B), and each a negative and positive example of pancreatic adenocarcinoma (C,D), pulmonary adenocarcinoma (E,F) and invasive breast carcinoma (G,H), respectively. Original magnification ×40. Scale bar: 20 µm.
Interestingly, while Alb-ISH was positive in more than two-thirds of all evaluable iCCA cases (positivity 69.5%, 105/151), cases diagnosed as pCCA were determined to be almost exclusively negative, translating to a positivity rate of only 1.3% (2/153) analogous to non-hepatic adenocarcinomas. Among the Alb-ISH positive iCCA cases, we observed a wide range of percentage of positive cells per case with some samples showing albumin expression in only a minor fraction (minimal 5%) of tumour cells and other cases demonstrating ubiquitous global expression patterns (100%). When calculated on all analysed samples as denominator (excluding pCCA), Alb-ISH specificity for iCCA was 98.8%.
Positive Alb-ISH is associated with small duct morphology in iCCA
Owing to the morphological and molecular similarities between pCCA and large duct iCCA, we investigated whether albumin negativity observed in pCCA might be mirrored also in large duct iCCA. Histopathological subtyping of all iCCA cases according to the current WHO criteria yielded a predominant proportion of 60.1% small duct morphology (92/153) outweighing large duct type iCCA with a frequency of 39.9% (61/153). Perineural invasion, as a formal criterion for subtyping, was nearly three times more frequent in large duct than in small duct iCCA (42.6% vs 15.2%, p=0.002, Fisher’s exact test) and was also associated with tumour size (19.1% vs 44.2%, T1–2 vs T3–4, p=0.002, Fisher’s exact test). Subtype-stratified contingency analysis revealed positive Alb-ISH to be highly associated with small duct morphology, mirrored by a significantly higher frequency of Alb-ISH positivity in small duct (81.3%, 74/91) as compared with large duct iCCA (51.7%, 31/60; p=0.001, Fisher’s exact test) (figure 2). Among the positive cases, there was no significant difference in the quantity of positive cells between small and large duct type iCCA (median 50% vs 60%; p=0.609, Mann-Whitney U test).
Albumin in situ hybridisation (Alb-ISH) in small/large duct intrahepatic cholangiocarcinoma (iCCA). Representative H&E-stained microphotographs of small duct iCCA (A) composed of irregular, small glands lined by cuboidal epithelium and large duct iCCA (B) with columnar cell morphology and mucin production. Correspondingly, an example of small duct iCCA with positive Alb-ISH (C) is contrasted with a large duct iCCA case negative for Alb-ISH (D). Original magnifications: (A–C): ×20 and (D): ×40. Scale bars: (A–C): 50 µm and (D): 20 µm.
CRP is a sensitive, but less specific marker for iCCA
Considering the clear-cut specificity of Alb-ISH for iCCA, we empirically defined all cases with any CRP immunoreactivity (IRS≥1) as positive to provide a second marker with maximised sensitivity. As such, 127 of all 151 evaluable iCCA cases were found positive for CRP, corresponding to a sensitivity of 84.1%. In iCCA, although CRP immunohistochemistry mostly showed a strong, cytoplasmic granular staining pattern in the majority of tumour cells with a median IRS of 8 among positive samples, a small fraction of 7.9% iCCA cases only exhibited focal CRP immunoreactivity in less than 20% of the tumour cells (figure 3). Similar to Alb-ISH, CRP positivity was significantly associated with small duct type morphology (sensitivities for small vs large duct iCCA 92.3% vs 71.7%, respectively, p=0.001, Fisher’s exact test). Furthermore, CRP immunoreactivity was highly associated with positive Alb-ISH (p<0.0001, Fisher’s exact test).
C reactive protein (CRP) immunohistochemistry in intrahepatic cholangiocarcinoma (iCCA). CRP staining in iCCA was scored semiquantitatively using the immunoreactive score (IRS) ranging from 0 (no expression) to 12 (high expression), integrating the percentage of positive tumour cells and staining intensity. Immunoreactivity of CRP in iCCA ranged from complete negativity ((A), IRS=0) over moderate expression ((B), IRS=4) to very strong, ubiquitous staining ((C), IRS=12). Original magnification: ×20. Scale bar: 50 µm.
In pCCA, CRP immunoreactivity was detected in 33.1% (49/148) of all samples when using the same minimal threshold. However, in contrast to iCCA the signal was mostly weak to moderate with a median IRS of 2. Interestingly, the two only Alb-ISH positive pCCA cases were also positive for CRP and displayed strong immunoreactivity (IRS=6 and IRS=12, respectively).
Among other adenocarcinomas (figure 4), colorectal liver metastases were vastly negative for CRP corresponding to a positivity rate of 11.6% (10/86) with immunoreactivity in only a minority of tumour cells and predominantly faint staining intensity (median IRS=2 among positive samples). Comparably low positivity rates were also observed in pancreatic ductal adenocarcinoma (9/80, 11.3%, median IRS=3 among positive samples), pulmonary adenocarcinoma (14/70, 20%, median IRS=3.5 among positive samples) and invasive breast carcinoma (8/212, 3.8%, median IRS=2 among positive samples). Frequency of immunohistochemical CRP expression was 29.4% in gallbladder cancer (35/119, median IRS=2 among positive samples) and 14.3% in dCCA (18/126, median IRS=2 among positive samples). Expression patterns of the diagnostic markers between iCCA and all other adenocarcinomas, as well as between small and large duct iCCA, are visualised in comparative violin plots (online supplemental file 1), highlighting the clear-cut difference between iCCA and all other adenocarcinoma entities (p<0.0001 for each comparison, Kruskal-Wallis test), and between small and large duct iCCA (p=0.027, Mann-Whitney U test). Excluding pCCA, specificity of CRP immunohistochemistry for a diagnosis of iCCA reached 86.4%. Raising the threshold to IRS=2 led to only a slight improvement in specificity (86.7%), although with a relatively higher decrease in sensitivity (83.4%) (online supplemental table 2).
C reactive protein (CRP) immunohistochemistry in adenocarcinomas of various sites. CRP expression was absent in skeletal muscle ((A), negative control) and ubiquitous in normal liver tissue ((B), positive control). Adenocarcinomas other than intrahepatic cholangiocarcinoma were predominantly negative for CRP and mostly displayed weak-to-moderate staining when positive, comprising colorectal liver metastasis (C,D), pancreatic adenocarcinoma (E,F), pulmonary adenocarcinoma (G,H) and invasive breast carcinoma (I,J). Original magnification: ×40. Scale bar: 20 µm.
Combined analysis of Alb-ISH and CRP immunohistochemistry augments diagnostic sensitivity for iCCA
Next, we assessed the potential of combined analysis of Alb-ISH and CRP immunohistochemistry for an iCCA diagnosis. Given the near-perfect specificity of Alb-ISH for iCCA, positivity of a single marker was considered sufficient for an iCCA diagnosis in the pooled analysis. Subsequently, a diagnosis of iCCA was only rejected in case of negativity for both Alb-ISH and CRP immunohistochemistry. Strikingly, integration of Alb-ISH and CRP immunohistochemistry boosted the sensitivity for a diagnosis of iCCA to 88.0% with only 18 false-negative cases (out of 150 combined-evaluable cases), while retaining a considerable specificity of 86.1%. Sensitivities and specificities for isolated Alb-ISH and CRP analysis as well as in the combined evaluation are summarised in table 1 (CRP threshold IRS=1) and online supplemental table 2 (CRP threshold IRS=2), respectively.
Metrics for Alb-ISH and CRP immunohistochemistry (threshold immunoreactive score=1) as diagnostic markers for iCCA
Alb-ISH is associated with tumour morphology and stage, but not patient survival
To assess whether Alb-ISH results may reflect distinct clinical or morphological phenotypes in iCCA, we compared positive and negative cases with respect to available clinicopathological information (table 2). Contingency analysis revealed a significant association of Alb-ISH with tumour histotyping, showing a solid tumour morphology to be more prevalent in Alb-ISH positive cases (p=0.029). Mucinous and signet ring cell differentiation were on the contrary exclusively found in the Alb-ISH negative group. In addition, we observed that Alb-ISH negative cases showed higher pathological tumour (pT) stages (p=0.049), which was paralleled by a trend towards more advanced Union for International Cancer Control (UICC) stages (p=0.063).
Clinicopathological characteristics of intrahepatic cholangiocarcinoma patients stratified for Alb-ISH
Highlighting the strong association with CRP immunohistochemistry, CRP-stratified analysis of clinicopathological criteria yielded analogous results with significant differences for UICC stage (p=0.047) and a trend towards inhomogeneously distributed morphologies (p=0.066) (table 3). Moreover, we found that vascular invasion (V1) was significantly more frequent among CRP-positive cases (p=0.049). Both for Alb-ISH and CRP immunohistochemistry, no significant differences with respect to age, sex, nodal stage, distant metastasis, tumour grading, residual tumour, lymphatic vessel invasion or perineural infiltration were detected.
Clinicopathological characteristics of intrahepatic cholangiocarcinoma patients stratified for CRP immunohistochemistry
Survival data were available for a subset of 131 iCCA patients. Kaplan-Meier survival analyses (figure 5) showed no significant differences in prognosis between Alb-ISH positive and Alb-ISH negative cases (median survival 4.2 years vs 3.8 years, p=0.83, log-rank test), nor after stratification for CRP (median survival 5.2 years vs 3.2 years, p=0.45, log-rank test).
Kaplan-Meier survival analysis on intrahepatic cholangiocarcinoma patients stratified for Alb-ISH or CRP immunohistochemistry. Survival curves did not differ between positive and negative groups for both Alb-ISH ((A), n=131, p=0.83) and CRP ((B), n=131, p=0.45). Median survivals: Alb-ISH positive 4.2 years, Alb-ISH negative 3.8 years; CRP positive 5.2 years, CRP negative 3.2 years. P values were calculated using log-rank test. Alb-ISH, albumin in situ hybridisation; CRP, C reactive protein; neg., negative; pos., positive.
Alb-ISH is associated with distinct molecular alterations
To assess whether Alb-ISH may be a prescreening parameter for predictive molecular alterations in iCCA, we correlated Alb-ISH with panel-based and whole exome-based sequencing data determined previously.21 Mutation data were available for a subset of 41 iCCA patients, of which 35 were Alb-ISH positive and 6 were Alb-ISH negative. Although the limited sample size precludes a generalised conclusion, of note, apart from one IDH1-mutated negative case with negativity for Alb-ISH, iCCA-prototypical mutations, such as IDH1 (n=13), IDH2 (n=2) and FGFR2 (n=2), were exclusively found in the Alb-ISH positive tumour subset (figure 6). In contrast, the only KRAS mutation observed corresponded to one of the six Alb-ISH negative cases with large duct type iCCA morphology. A similar pattern was observed for CRP, with the presence of the afore-mentioned prototypical mutations in almost half of all evaluable CRP-positive cases (17/36), but complete absence in CRP-negative cases (0/4).
Distribution of recurrent mutations among albumin in situ hybridisation (Alb-ISH) positive and negative intrahepatic cholangiocarcinoma (iCCA). Alluvial plot showing the distribution of prototypical iCCA mutations (IDH1, FGFR2, IDH2 and KRAS) among the iCCA cases with available mutation data (Alb-ISH positive n=35, Alb-ISH negative n=6).
Discussion
Due to the absence of established specific markers, iCCA is a diagnosis of exclusion, even for the diagnostic pathologist. Although immunohistochemistry is helpful in the diagnostic process, the immunoprofile can be indistinguishable from other primary tumours, such as pancreatic adenocarcinoma, resulting in a level of uncertainty of classification even after thorough clinical, imaging and histopathological workup in a significant number of cases.22 However, since clinical decision-making (eg, systemic therapy) heavily relies on correct tumour typing, specific markers for iCCA diagnosis are of high diagnostic relevance. We therefore assessed the combined use of two promising diagnostic modalities, Alb-ISH and CRP immunohistochemistry, for iCCA diagnosis on a comprehensive European iCCA cohort and additionally tested more than 800 cases of other adenocarcinomas originating from various locations. Our results identified Alb-ISH to be highly specific for iCCA with only single false-positive cases among non-hepatic adenocarcinomas at a sensitivity of roughly 70%. In contrast, CRP immunohistochemistry showed a relatively high sensitivity for iCCA reaching 84.1% at the cost of more false-positive classifications among adenocarcinomas of other sites.
Although this is to date the by far largest study on the utility of Alb-ISH in the diagnostic setup for liver cancer, other studies have reported on this topic before with substantial variance regarding specificity and sensitivity. In fact, reported sensitivities of Alb-ISH for iCCA ranged from 63% up to almost 100%.12 14–17 Possible reasons for this discrepancy are attributable to differences in patient populations, putative misclassifications of biliary tract cancer subtypes and methodology. As shown in this study, small duct iCCA is significantly more likely to express albumin than large duct iCCA. Hence, a small duct dominated iCCA cohort may show entirely different positivity rates than a cohort with a relevant number of large duct iCCA. In addition, particularly due to the significant background signal of the adjacent liver tissue, it may be possible that overinterpretation of focal signal presence may lead to aberrant results in borderline cases. The clear-cut specificity of Alb-ISH for iCCA is in line with most previous studies, which even demonstrated total absence of Alb-ISH signal in adenocarcinomas of other sites.12 14–17 With respect to CRP immunohistochemistry, the metrics derived in this study are compatible with the literature reporting sensitivities of 62%–93% and specificities of 88%–95%.8 11 A combined analysis of Alb-ISH and CRP immunohistochemistry boosted sensitivity for an iCCA diagnosis to 88.0% at a considerable specificity of 86.1%, which remained virtually unchanged compared with the isolated CRP analysis.
For routine implementation, we propose a sequential approach as illustrated in figure 7. In cases with pancreatobiliary morphology and compatible cytokeratin immunophenotype, owing to the high sensitivity we advocate widely available and less expensive CRP immunohistochemistry as initial step to substantiate the diagnosis of iCCA at adequate certainty. Sequentially, if there is still clinical doubt about the diagnosis or in case of negative CRP immunohistochemistry, Alb-ISH should be considered. Through this case-dependent and easy-to-implement approach, diagnostic certainty can be increased significantly under limited additional test and labour-related expenditure.
iCCA diagnostic algorithm based on CRP immunohistochemistry and Alb-ISH. Alb-ISH, albumin in situ hybridisation; CRP, C reactive protein; iCCA, intrahepatic cholangiocarcinoma; IHC, immunohistochemistry.
Although Alb-ISH and CRP may be helpful tools for pathological subtyping of iCCA, subtype-specific evaluation of both assays is limited to two studies, each addressing one of the markers. While Sigel et al identified almost 1 out of 5 large duct iCCAs to be positive for Alb-ISH, Akita et al reported only 1 out of 19 large duct cases to express CRP immunohistochemically.8 15 These frequencies are remarkably lower than in the current study and stand in contradiction with the general perception of an increased sensitivity of CRP over the highly specific Alb-ISH towards a diagnosis of iCCA. Several factors may contribute to the observed discrepancies. First of all, we used a minimal IRS of 1 as a threshold for CRP to maximise sensitivity, that is, any cytoplasmic staining was considered a positive result. Otherwise, as evident from the expression distribution due to the presence of many cases with only focal staining, a significant proportion of the cases must have been excluded. Moreover, although the WHO published consensus criteria for subtyping in 2019, the applied definitions vary among previous investigations.18 For instance, in the study by Sigel et al, mucin production was disregarded as a feature of large duct iCCA, and tumours involving hilar/perihilar regions were entirely excluded.15 This discrepancy may not only add to different results in marker expression but also contribute to the high variance in subtype frequencies reported in the literature, ranging from 8% to 59% with the current study aligning closely with this spectrum.5–7 15 23 In addition, as confirmed in this study, morphological criteria, such as perineural or lymphatic invasion, are not absolute, but may also depend on specific characteristics of the study cohort, including tumour sizes. Epidemiological factors, such as chronic viral hepatitis or hepatic lithiasis, are to be considered here as well.5 24 Last but not least, subtype-specific differences may have also been impacted by the usage of different antibodies, RNA probes or general assay design.
Our data support the view, that large duct iCCA and pCCA share morphological and biological features, but are not identical. As discussed before, large duct iCCA and pCCA likely originate from the lining epithelium of large bile ducts and/or may arise from peribiliary glands. However, to date it is not known, which specific cell of origin is associated with each location and at what frequency.24 Considering the challenges that arise from the current anatomic definitions and the emerging relevance of targeted therapy, in our eyes there is justification to refine the existing classification. We propose Alb-ISH as an additional modality to molecularly stratify CCAs conventionally partitioned into iCCA and pCCA. Although the anatomy-based distinction between iCCA and pCCA is still of enormous relevance to surgeons and needed for correct tumour staging, a parallel classification scheme employing immunohistochemical and molecular testing algorithms for small and large duct iCCA would avoid misclassification and better align with the demands of continuously evolving personalised therapy.
A limitation of this study is the usage of TMAs as an efficient way to parallelize a double-digit number of immunohistochemical analyses on one histological section. Naturally, dependent on the grade of heterogeneity of the marker, evaluation on whole slides may yield different results since positive cases may be missed on the tissue dots provided in the TMA. To address this issue, in all TMAs used in this study per each sample, two tissue cores of different tumour areas were employed. Moreover, meta analyses objectified a high rate of agreement for all kinds of immunohistochemical markers between TMA and whole slide evaluation with concordance rates ranging from 80% to 100%.25 26 Lastly, since initial diagnoses are predominantly made on small biopsies, usage of TMA may even be considered to better replicate the clinical situation with the possibility of small sample size in tumour biopsies, for instance.
In summary, we here assessed the combined potential of Alb-ISH and CRP immunohistochemistry as diagnostic markers for iCCA on a large iCCA patient cohort, counter checked for more than 800 samples of other adenocarcinoma types and thereby providing a sequential diagnostic algorithm to be implemented in routine diagnostic pathology. Furthermore, based on a strong association between Alb-ISH positivity and underlying molecular alterations, future studies should delineate whether Alb-ISH positive CCA and Alb-ISH negative CCA can be clearly separated on the clinical and molecular level and therefore may be helpful as distinct groups with implications for treatment options.
Data availability statement
Data are available upon reasonable request. The datasets generated during and/or analysed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by ethics committee of the Medical Faculty of Heidelberg University (S-206/2005 and S-519/2019). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors thank Nina Wilhelm and Veronika Eckel (tissue bank of the NCT Heidelberg, Germany) for excellent technical assistance. Samples were provided by the tissue bank of the NCT Heidelberg (Germany) in accordance with the regulations of the tissue bank and the approval of the ethics committee of the Medical Faculty of Heidelberg University.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Handling editor Yoh Zen.
Contributors TA: conceptualisation, data analysis, methodology and original draft writing. AR: resources and validation. FR: methodology. KB: methodology and review. E-MB: sample collection and data analysis. MT: methodology and review. FB: data analysis and resources. AC: data analysis and resources. MNV: sample collection and review. BK: resources and editing. AM: resources and editing. MWB: resources and review. SS: data analysis. WS: editing. BS: data analysis. PS: supervision, review and editing. SR: supervision, data analysis, review and editing. BG: initiation of the study, conceptualisation, sample collection, data analysis, original draft writing, reviewing and editing. All authors have read and agreed with the final manuscript. BG accepted full responsibility as guarantor for the study and/or the conducted of the study, had access to the data and controlled the decision to publish.
Funding This work was supported by the German Research Foundation (project-ID 314905040—SFB/TRR 209 Liver Cancer) and the European Union’s Horizon 2020 research and innovation programme under Eurostars (grant E! 113707, LiverQR) to PS and SR. SR received additional funding from the German Research Foundation (project no. 469332207 and project no. 493697503) and German Cancer Aid (project no. 70113922). TA was supported by the Physician-Scientist programme of the Medical Faculty of Heidelberg University.
Competing interests PS: advisory board at Incyte, BMS, MSD, Eisai, Janssen, Bayer, Roche, Novartis; speakers bureau at Incyte, BMS Janssen; research grants from Incyte, BMS, Novartis, Chugai, Brunker. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results. The other authors declare no competing interests.
Provenance and peer review Not commissioned; internally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.